澳大利亚TGA认证
澳大利亚TGA全称是The Therapeutic Goods Administration,是澳大利亚政府卫生部的一部分,负责管理治疗用品,包括处方药、疫苗、防晒霜、维生素和矿物质、医疗设备、血液和血液制品。几乎任何声称具有治疗功效的产品都必须被登记在澳大利亚ARTG,然后才能在澳大利亚供应。
The Therapeutic Goods Administration (TGA) is part of the Australian Government Department of Health, and is responsible for regulating therapeutic goods including prescription medicines, vaccines, sunscreens, vitamins and minerals, medical devices, blood and blood products. Almost any product for which therapeutic claims are made must be entered in the Australian Register of Therapeutic Goods (ARTG) before it can be supplied in Australia.
医械产品分类确定
The manufacturer is responsible for determining the classification of a device using a set of classification rules based on the:
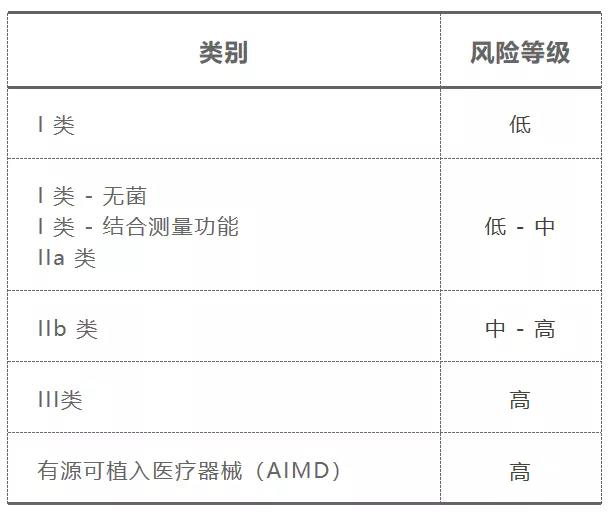
1.医疗器械的预期用途。
·manufacturer’s intended use of the device
2.对病人,用户及其他人的风险等级。
·level of risk to patients, users and other persons (the probability of occurrence of harm and the severity of that harm)
3.植入人体的程度。
·degree of invasiveness in the human body
4.使用时限。
·duration of use
其分类等级划分如下表:
符合性评估
什么是符合性评估?
制造商必须能够证明该器械以及制造该器械的过程符合澳大利亚相关法规的要求。
相关法规如下:
Therapeutic Goods Act 1989 (the Act)
Therapeutic Goods (Medical Devices) Regulations 2002 (the Regulations)
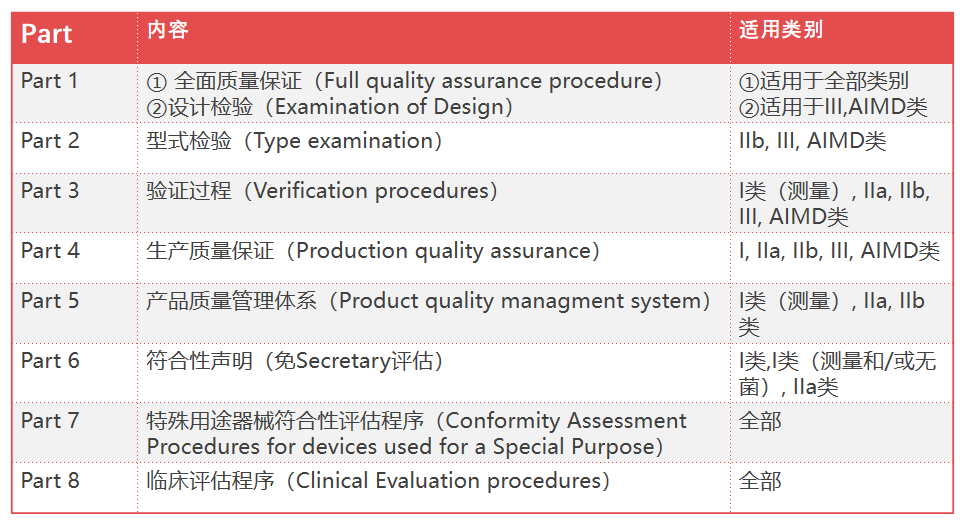
符合性评估证据类型:
The TGA accepts the following certificates as conformity assessment evidence:
-TGA颁发的符合性评估证书(对部分企业是唯一途径)
a TGA Conformity Assessment Certificate issued by the TGA - this is mandatory for some manufacturers
-澳大利亚EC MRA 符合性证书
certificates of conformity issued under the Australia -EC MRA
-澳大利亚 EFTA MRA 符合性证书
certificates of conformity issued under the Australia -EFTA MRA
-由欧盟公告机构颁发的EC证书
EC certificates issued by an EU Notified Body
符合性评估所需材料:
选择微珂的理由:
微珂医药拥有海内外专业成熟的技术团队、以及与机构深度的战略合作,服务于医疗器械企业,为企业提供优质的定制化服务,协助企业从产品技术要求编写、产品检测、临床评价资料编写与审核、申报与跟踪,根据企业实际情况,进行质量管理体系建立,帮忙企业从管理到产品各环节一站式服务!



地址 :上海市静安区平陆路889号(上海静安华发中心)901室
邮件 :owen.he@microkn.com
手机 :18017580586
